The Developmental Therapeutics Core (DTC) helps investigators evaluate promising novel therapeutic agents and diagnostics, as well as existing therapeutics being considered for novel uses (repurposing), through continued development and deployment of relevant animal models and in vitro cell lines and tissue models. We provide several services to investigators to suit many needs, including those who may lack laboratory facilities and/or expertise for conducting experiments associated with preclinical therapeutic hypothesis testing
Services

Cancer Research
The Developmental Therapeutics Core offers a comprehensive range of services to support oncology research, from in vitro assays to advanced in vivo models. For in vitro studies, over 160 cancer cell lines are available for drug testing, including IC50 determination, as well as assays for cell proliferation and apoptosis. Researchers can also assess therapeutic effects in 3D or organoid cultures, providing a more physiologically relevant environment. In vivo cancer models include subcutaneous and orthotopic xenografts, syngeneic mouse models, and studies of tumor metastasis. Tumor growth is monitored through various techniques, including Biopticon TumorImager-TumorManager, caliper measurements, and IVIS imaging. Surgical services, such as ovariectomy and castration, are offered, and the core also provides assistance with patient-derived xenografts (PDX) and the development of humanized mouse models. Additionally, efficacy experiments are conducted to test the effects of novel therapeutics, offering a robust platform for preclinical evaluation of cancer treatments.

Therapy-Response Experiments
Our personnel provide expert guidance in determining the most appropriate models for evaluating the efficacy of compounds and biologicals of interest. Services include experimental design consultation, animal procurement, and execution of the experiment according to the approved protocol. We work closely with cores such as the Center for Advanced Molecular Imaging (CAMI), Center for Translational Imaging (CTI), Integrated Molecular Structure Education and Research Center (IMSERC), Mouse Histology and Phenotyping Laboratory (MHPL), and The Behavioral Phenotyping Core (BPC) to address all aspects of a study as needed.

Reproductive Research
Core provides a wide array of services to support studies on reproductive health and fertility. Services include estrous cycle monitoring and vaginal plug evaluation to assess mating timing and success. Researchers can analyze mating success, pregnancy rates, and perform behavioral observations, such as mating and nesting behaviors. The core offers assessments of embryo implantation sites and fetal resorption rates, which are essential for studying early pregnancy outcomes. Additionally, the core can collect blood samples and reproductive organs for further analysis. Ovarian follicle and corpus luteum counts are performed to evaluate ovarian function, while sperm morphology, count, and motility assessments provide insights into male fertility. These services offer a thorough foundation for investigating reproductive processes and potential impacts on fertility.
Exploratory PK and Tox
Exploratory pharmacokinetics analysis can be incorporated into therapy-response experiments through organ and tissue harvest of treated animal subjects at pre-determined time points during treatment. Many projects require and include determination of therapeutic maximum tolerated dose (MTD) prior to initiating a therapy-response experiment with 7-day Repeated Dose also offered. We work collaboratively with the Integrated Molecular Structure Education and Research Center (IMSERC) to provide full pharmacokinetics analysis as well as with the Mouse Histology and Phenotyping Core (MHPL) to evaluate treated organs and tissues for toxicity.

Device Implantation and Monitoring
We collaborate with investigators to implant and evaluate new devices in vivo, following the submission and approval of an IACUC protocol for surgical implantation. The core can then assess the device for toxicity and function and/or work with other cores to obtain additional imaging modalities.

Immunization
DTC has the capability to produce monoclonal antibodies from peptides in both mice and rabbits.
Project Workflow
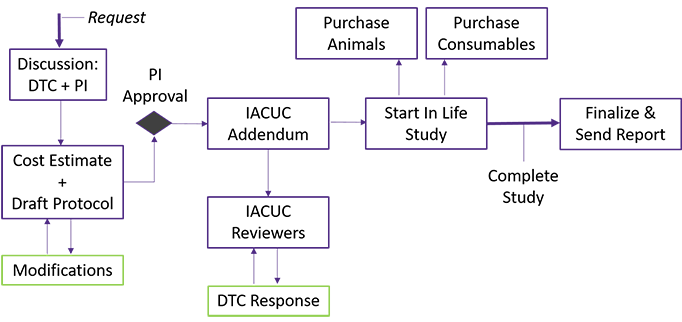
We provide an initial consultation regarding model selection, experimental design, and types of analyses to be performed. A study protocol and cost estimate are generated and approved by the user. At the completion of the study, data is collected and analyzed with a final report furnished in 1-2 weeks.
PLEASE NOTE: Our core maintains several blanket ACU protocols to fit various user needs. This study cannot begin without ACU approval.